English
Integrated solution
Preclinical pharmacology encompasses a range of studies, including pharmacodynamic research, pharmacokinetic studies, pharmacological evaluations, and toxicological safety assessments. These studies are essential to drug evaluation and are used to determine the efficacy of investigational drugs, identify appropriate effective and safe dosing regimens, elucidate the characteristics of investigational drugs, and uncover potential mechanisms of action. In addition, pharmacology studies can supplement clinical therapy and demonstrate the rationality of drug combinations.
The PK Testing Platform focuses on pharmacokinetic testing and analysis of antibody-based therapeutics. Our main technical platforms include ELISA, MSD (electrochemiluminescence), and quantitative pharmacological modeling analysis software WinNolin. We provide quantitative detection method development and validation services that meet the guidance principles of the NMPA and the FDA. We offer efficient and stable blood drug concentration screening for monoclonal and bispecific antibodies, as well as pharmacokinetic parameter analysis to support candidate molecule evaluation, experiment design, and clinical translational research.
Biological activity determination :ADCC/ADCP/CDC, cell proliferation/inhibition detection, cell killing activity verification, neutralization activity verification , cell apoptosis detection, cytokine release test , antibody internalization, etc.
Our in vitro pharmacology platform utilizes primary cells and cell lines to evaluate key in vitro pharmacological parameters of candidate antibodies, including binding affinity, Fc/Fab-related functionality, etc. Our experimental techniques include flow cytometry, ELISA, HTRF, and Incucyte cell imaging. Our platform supports the elucidation of candidate antibody efficacy and toxicity mechanisms and facilitates clinical translational research.
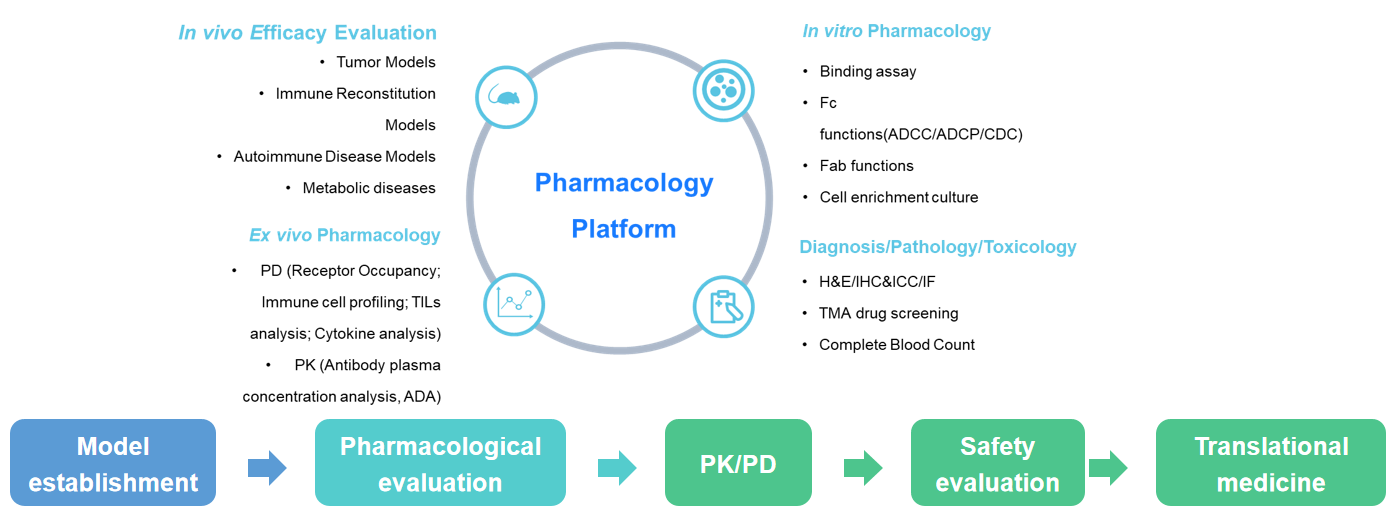
- In vivo pharmacodynamic evaluation: tumor model/immune reconstitution model/auto-immune disease/inflammatory model metabolic model.
Based on our independently developed humanized mouse models for immune checkpoint, cytokine, and its receptor, as well as our severe immunodeficiency mouse strain B-NDG and its offspring, BioMice possesses expertise in tumor, autoimmune, metabolic, and immune-reconstituted modeling technologies. We offer customized in vivo drug efficacy testing solutions for different target types, antibody subtypes, and mechanisms of action. We conduct various experiments and provide diverse analysis data to accelerate preclinical drug discovery and development processes for our customers.
- In vivo pharmacological evaluation: in vivo pharmacokinetic studies/receptor occupancy analysis/TILs analysis/cytokine analysis, etc.
- In vitro efficacy evaluation services
Biological activity determination :ADCC/ADCP/CDC, cell proliferation/inhibition detection, cell killing activity verification, neutralization activity verification , cell apoptosis detection, cytokine release test , antibody internalization, etc.
- Pathology/Toxicology testing services






 +86-10-56967680
+86-10-56967680 info@bbctg.com.cn
info@bbctg.com.cn